Zoltán Varga and Attila
Kovács
Hydrogen
bonding in peptide secondary structures.
International Journal of Quantum Chemistry, 105 (2005) 302-312
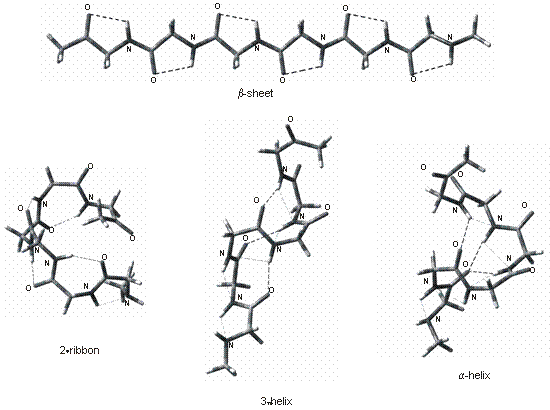
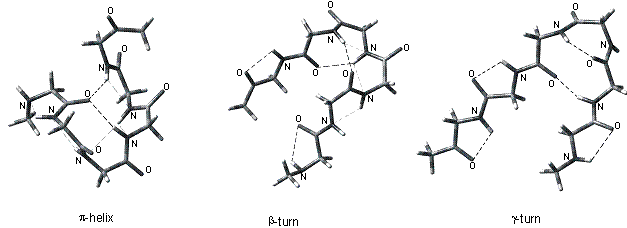
Hydrogen bonding interactions
in various peptide secondary structures (b-sheet,
27-ribbon, 310-helix, a-helix,
p-helix, b-turn
II and g-turn) have been investigated
in small oligopeptides by quantum chemical
calculations at the B3LYP/6-31G** level. Beside the primary O…H–N
interactions the optimized structures revealed the importance of N…H–N
hydrogen bonding in several structures. The effect of substitution on the
energy and structural properties was investigated comparing the properties of glycine, alanine, valine and serine. The aliphatic substituents
weaken generally the hydrogen bonds, the strongest effects being observed in
crowded valine conformers. Additional hydrogen
bonding interactions introduced by the OH group of serine can both strengthen
(by polarizing the amide moiety through N…H interaction) and
weaken (constraining the C=O oxygen by O…H–O interaction)
the backbone hydrogen bonds. The effect of water as a polarizable
medium on the energy properties was assessed by the COSMO model.