Balázs Nyerges and Attila Kovács
Density
Functional study of the conformational space of 4C1 d-glucuronic acid
The Journal of Physical Chemistry A, 109 (2005) 892-897
The conformational space of 4C1
a- and b-D-glucuronic acid was scanned by HF/3-21G(p)
calculations followed by optimization of the 15 most stable structures for
each, using the B3LYP density functional theory method in conjunction with a
diffuse polarized valence triple-zeta basis set. We found a general preference
of the a anomers in the isolated molecules in
agreement with the large endo-anomeric hyperconjugation effects in these
structures. From the other intramolecular interactions (exo-anomeric
hyperconjugation, hydrogen bonding, dipole-dipole, and steric interactions) the
effect of the hydrogen bonding is the most pronounced and plays a major role in
determining the stability order within the a and b
series. The most stable conformer of both a and b 4C1
D-glucuronic acid is the structure with the maximum number (5) of
intramolecular hydrogen bonds. Introduction of solvent (water) effects by the
SCI-PCM model resulted in two characteristic changes of the energetic
properties: the gas-phase stability order changed considerably and the energy
range of the 15 most stable conformers decreased from 30 kJ/mol to 15 kJ/mol.
The geometrical parameters reflect well the superimposed effects of
hyperconjugation and hydrogen bonding interactions. Most characteristic are the
variations of the C–O bond distances (within a range of 0.04 Å)
upon the combined intramolecular effects.
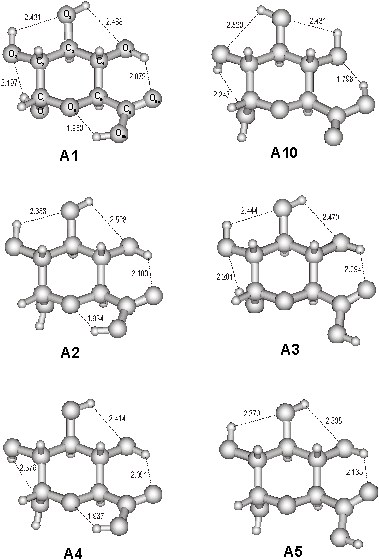
Selected
conformers of a-d-glucuronic acid