Theoretical
study of hyaluronan disaccharides
The aim of our study is to determine the
characteristic structures of hyaluronan disaccharides
and their stabilizing factors. Two types of disaccharides were examined, the
β-D-glucuronic acid-(1->3)-β-D-N-acetylglucosamine (further acid-amin)
and β-D-N-acetylglucosamine-(1->4)-β-D-glucuronic acid (further amin-acid)
where both the free acids and anions, all together four different
constitutional isomers, were modeled. These disaccharides can exist in numerous
conformations determined by the hydrogen bonds formed. We searched for the most
stable conformers, which are shown in the tables below. These computations have
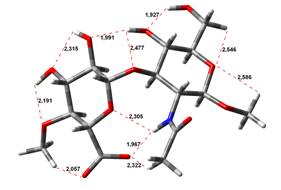
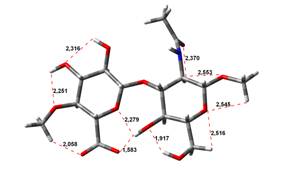
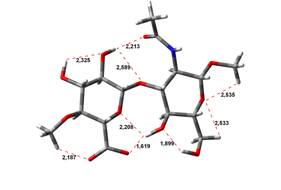
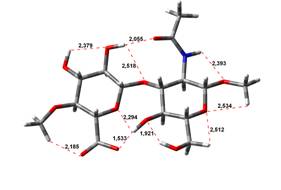
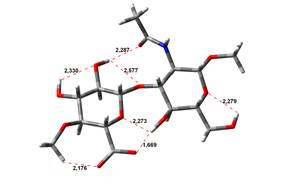
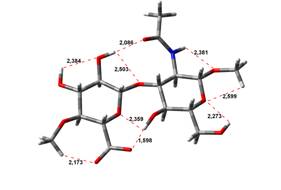
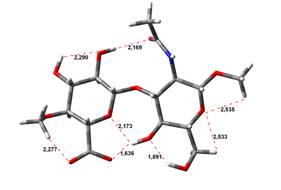
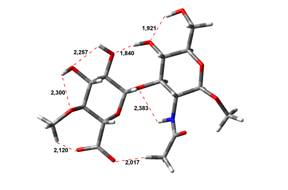
been done at the B3LYP/6-31G** level of theory. The hydrogen bond lengths are
given in angstroms, while the relative energies in kJ.mol-1.
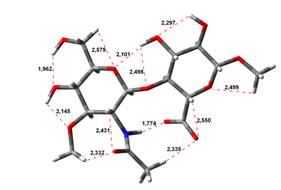
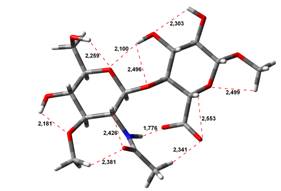
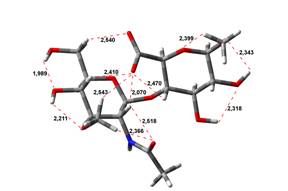
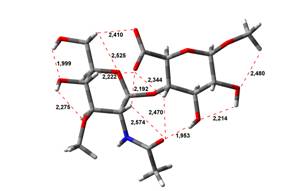
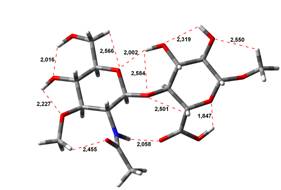
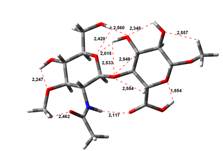
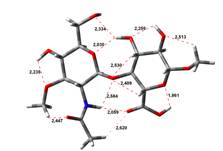
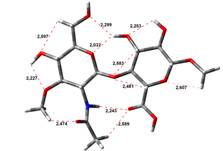
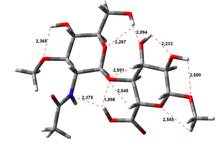
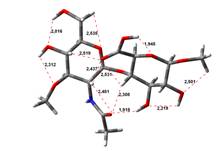
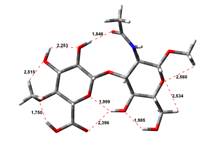
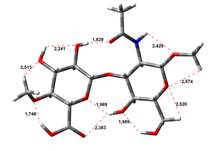
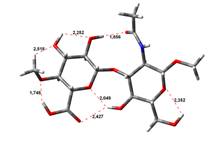
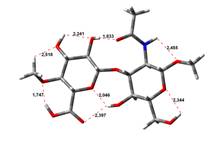
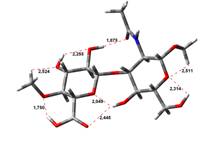
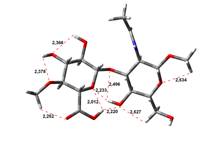
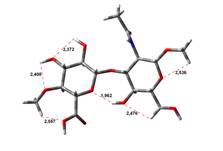
Amin-Acid structures
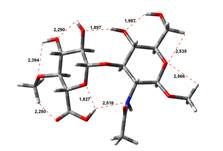
Anion form
|
|
|
|
0,00 kJ.mol-1 |
4,45 kJ.mol-1 |
|
|
|
|
13,97 kJ.mol-1 |
18,19 kJ.mol-1 |
|
|
|
|
92,92 kJ.mol-1 |
97,42 kJ.mol-1 |
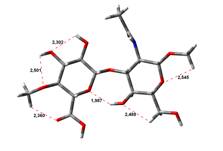
Free Acid form
|
|
|
|
0,00 kJ.mol-1 |
4,64 kJ.mol-1 |
|
|
|
|
5,58 kJ.mol-1 |
5,96 kJ.mol-1 |
|
|
|
|
7,07 kJ.mol-1 |
9,36 kJ.mol-1 |
|
|
|
|
12,17 kJ.mol-1 |
16,50 kJ.mol-1 |
|
|
|
|
20,50 kJ.mol-1 |
22,30 kJ.mol-1 |
|
|
|
|
30,17 kJ.mol-1 |
42,76 kJ.mol-1 |
|
|
|
|
47,25 kJ.mol-1 |
|
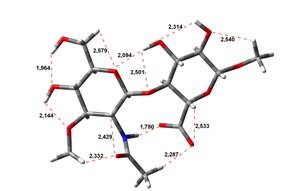
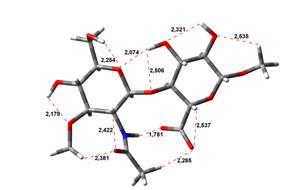
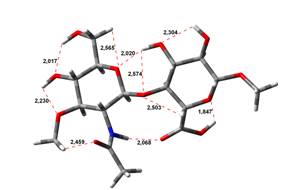
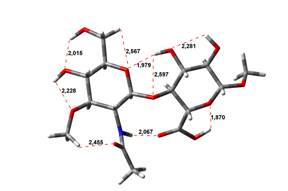
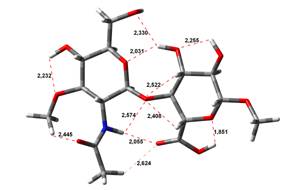
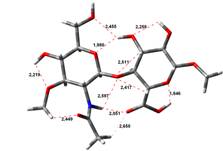
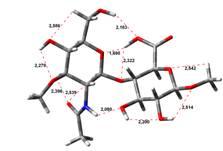
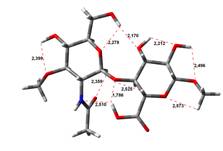
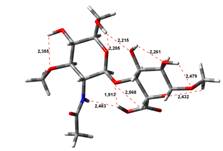
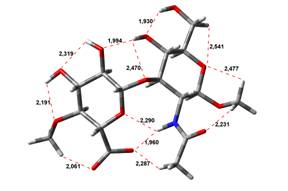
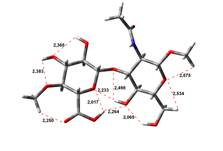
Acid-Amin structures
Anion form
|
|
|
|
0,00 kJ.mol-1 |
5,45 kJ.mol-1 |
|
|
|
|
14,51 kJ.mol-1 |
22,20 kJ.mol-1 |
|
|
|
|
27,14 kJ.mol-1 |
37,00 kJ.mol-1 |
|
|
|
|
41,26 kJ.mol-1 |
42,51 kJ.mol-1 |
|
|
|
|
62,58 kJ.mol-1 |
|
Free Acid form
|
|
|
|
0,00 kJ.mol-1 |
3,91 kJ.mol-1 |
|
|
|
|
6,56 kJ.mol-1 |
10,65 kJ.mol-1 |
|
|
|
|
11,65 kJ.mol-1 |
23,92 kJ.mol-1 |
|
|
|
|
32,29 kJ.mol-1 |
42,82 kJ.mol-1 |
|
|
|
|
45,07 kJ.mol-1 |
47,69 kJ.mol-1 |