Attila
Kovács, Gábor Keresztury, Vladislav Izvekov
Intramolecular hydrogen bonding in 2-nitrorezorcinol. A
combined
FT-IR, FT-Raman and computational study.
Chemical Physics, 253
(2000) 193-204
The vibrational properties of 2-nitroresorcinol
have been studied by a combined experimental and theoretical analysis. The
FT-IR and FT-Raman spectra of the compound have been recorded in the mid- and
far-IR range (4000-150 cm-1).
Symmetry related experimental information was obtained from polarisation IR and
Raman measurements. The interpretation of the spectra was aided by quantum
chemical calculations carried out at the B3-LYP/6-31G* level. Both the spectra
and calculations support the C2v structure of the molecule with a symmetric intramolecular
–O–H...O–N–O...H–O– hydrogen bonding interaction. The
deficiencies of the computations for the molecular vibrations were corrected by
the scaled quantum mechanical (SQM) method of Pulay. Using the standard scale
factors developed for B3-LYP/6-31G* force fields, an rms deviation of 9.7 cm-1
was achieved between the gas-phase experimental and SQM frequencies. As a
result of our SQM analysis, all the 42 fundamentals of the molecule were
assigned. Comparison with the analogous hydrogen bonding moiety in
2-nitrophenol indicates a somewhat stronger hydrogen bonding interaction in the
title molecule. This is accompanied, however, by an increased strain in the
six-membered –C–O–H...O–N–C– rings.
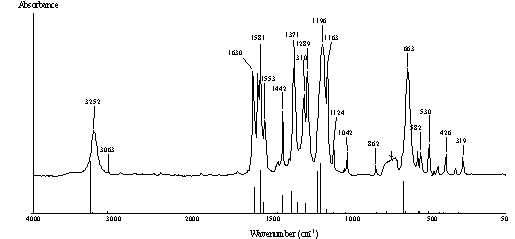
The experimental (solid) and computed IR spectra.