Attila Kovács, Katalin
Mészáros Szécsényi, Vukadin M. Leovac, Zoran D. Tomić, György Pokol
Synthesis
under self-controlled reaction conditions: reaction of tetraamminezinc(II)
chloride with 3,5-dimethyl-1-thiocarboxamide pyrazole
Journal of Organometallic Chemistry, 692 (2007) 2582-2592
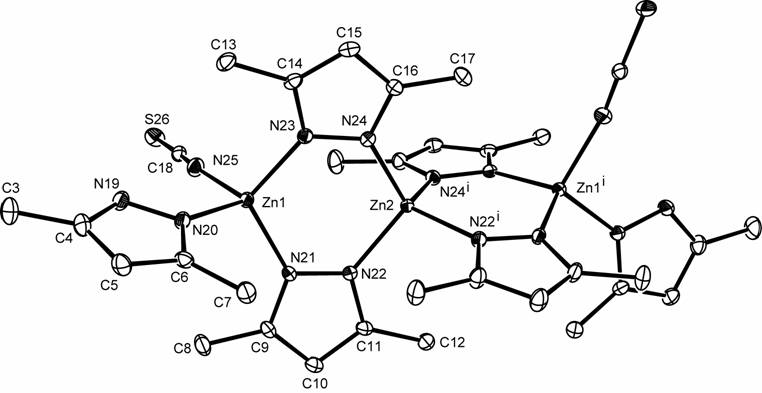
The trinuclear
[Zn3(Hdmpz)2(dmpz)4(NCS)2]
complex (Hdmpz: 3,5-dimethylpyrazole)
has been synthesized by the reaction of tetraamminezinc(II) chloride with
3,5-dimethyl-1-thiocarboxamidepyrazole. The ammonia evolving gradually from [Zn(NH3)4]Cl2 ensured
a mild gradually increasing basic pH during the synthesis which caused a
cleavage of the 1-N substituent. Moreover,
the changing pH controlled the pyrazolate anion - neutral ligand equilibrium,
and in this way the formation of the precipitate complex. The structure of the
complex was investigated by X-ray diffraction and quantum chemical computations.
The complex was characterized in detail by FT-IR-spectroscopy and thermal
analysis. The bonding interactions between Zn2+ and the ligands were
analysed on the basis of the computed data.