Andrea Szabó, V. I. Češljević, and Attila Kovács
Tautomerism, hydrogen
bonding and vibrational properties of 4-acetyl-3(5)-amino-5(3)-methylpyrazole.
Chemical Physics, 270
(2001) 67-78.
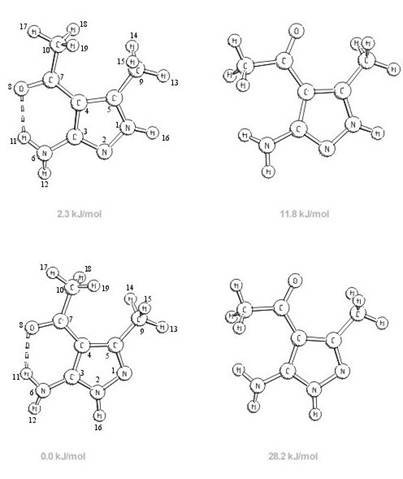
The structure and molecular
vibrations of 4-acetyl-3(5)-amino-5(3)-methylpyrazole have been investigated by
quantum chemical calculations and vibrational spectroscopy. The calculations
predicted a predominance of the 5-amino-3-methyl form in the tautomeric
equilibrium, however, the energy difference between the two tautomers is rather
small, 2 kJ/mol at the B3-LYP/6-311++G** level of the theory. The most stable conformers
of each tautomer are stabilized by intramolecular hydrogen bonding. This
interaction plays an important role also in the tautomeric equilibrium
contributing significantly to the stability of the 5-amino-3-methyl tautomer.
Another characteristics of the hydrogen bonding is its strengthening effect on
the conjugation of the NH2 and C=O groups with the pyrazole ring.
The molecular vibrations of the
title compound have been analysed by means of the scaled quantum mechanical
(SQM) method. Using joint theoretical and experimental information the FT-IR
and FT-Raman spectra of solid 4-acetyl-3-amino-5-methylpyrazole have been
assigned.