Attila Kovács
Theoretical study
of the strong intramolecular hydrogen bond and metal-ligand interactions in group 10 (Ni, Pd, Pt) bis(dimethylglyoximato) complexes
Journal of Organometallic
Chemistry,
692 (2007) 5383-5389
The structural and bonding
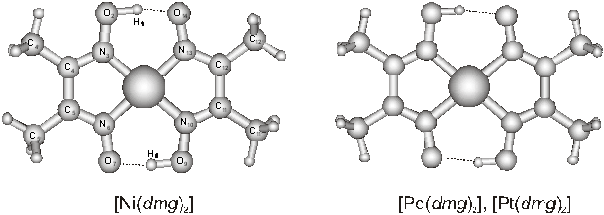
characteristics of the bis(dimethylglyoximato) complexes of
group 10 transition metals ([M(dmg)2],
where M = Ni, Pd and Pt) were investigated by means of quantum chemical computations.
The equilibrium geometries, energetic and bonding properties were computed
using the B3P86 exchange-correlation density functional in conjunction with a
6-311+(+)G** basis set. The computations revealed that
the strong O-…H–O hydrogen bond exists only in the presence of the
metal cations. The free (dmg)22- ligand
has significantly different geometry in which the O-…H–O
interaction is replaced by N…O–H bonds. The characteristics of the
metal-ligand interactions were determined by Natural Bond Orbital analysis.