A. Kovács, D. Nemcsok, G. Pokol, K.
Mészáros Szécsényi, V. M. Leovac, Ž. K.
Jaćimović, I. R. Evans, J. A. K. Howard, Z. D. Tomić, G. Giester
Structural,
spectroscopic and computational studies of the HgL2Cl2
complex (L=3,5-dimethyl-1-thiocarboxamide pyrazole) and the crystal structure
of L
New Journal of Chemistry, 29 (2005) 833-840
In the present paper we report the synthesis as
well as the structural and vibrational characterisation of the HgL2Cl2
complex (L = 3,5-dimethyl-1-thiocarboxamide). The crystal and molecular
structures of both L and the HgL2Cl2 complex were
determined by single-crystal X-ray diffraction. The coordination propensity of
L to HgCl2 was explored by quantum chemical calculations. We found
the preference of the monodentate coordination of L to HgCl2 through
the sulphur atom (instead of the “pyridine” nitrogen) in agreement
with Pearson’s acid-base character of the atoms involved and the steric
effects. The vibrational properties of HgL2Cl2 were evaluated
by a joint FT-IR and quantum chemical analysis. In addition, the thermal
decomposition of the complex and ligand is reported.
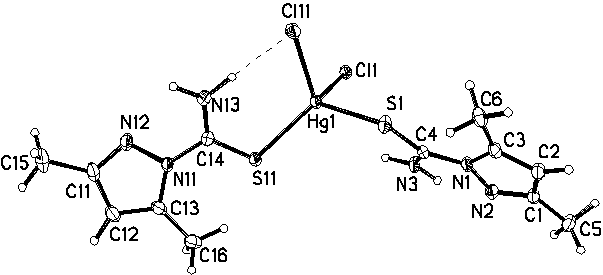
Molecular structure and intramolecular hydrogen bonding in HgL2Cl2
in the crystal.